Your life sciences partner throughout the entire product life cycle
The QbD Group supports life sciences companies worldwide from idea to patient.
Our Services
QbD’s team offers knowledge & tailored (software) solutions in development, clinical, regulatory & compliance, production and distribution for companies active in ATMP, Biotech, Medical Devices, In Vitro Diagnostics, Digital Health and Pharma.
Regulatory Affairs
We help our clients in their journey throughout the entire drug and medical device regulatory lifecycle.
Clinical
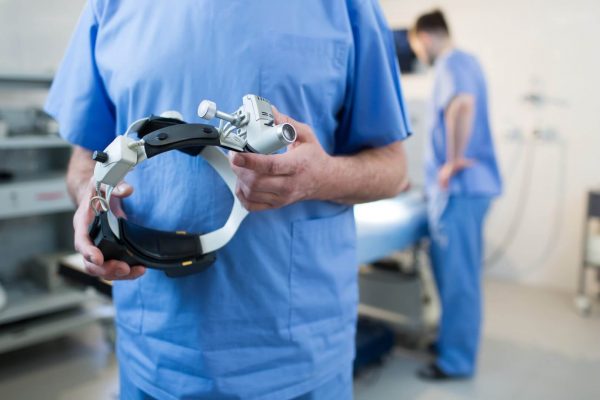
We are an expert clinical solutions provider specialized in medical devices and biotech, offering global CRO services and consultancy.
Qualification & Validation
We guarantee that products meet quality demands and comply with regulations through the qualification and validation of your equipment, facilities, and process support.
Quality Assurance
We offer the full range of QA services necessary to get your product to market in a safe and compliant way, including auditing from certified auditors, setting up your QMS, and all QP activities.
Lab Services
Our lab services division consists of a GMP laboratory that offers analytical services for testing pharmaceutical raw materials, excipients, and release testing of finished products.
Software Solutions & Services
Our solutions include a cloud-based and pre-validated QMS built for the life sciences, as well as eIFU services which enable IVD and MD manufacturers to digitalize paper Instructions For Use.
Our range of services include Computer System Validation and Digital Health services.
Business & Communications
We help you launch your product to market by providing marketing and communication services, business development support and sales strategy.
QbD offers a set of services globally called One Vigilance, with scientific methodologies, proprietary tools, and a team of experts in Pharmacovigilance, Biopharmacovigilance, and Medical Devices vigilance
Are you prepared to tackle the challenges of clinical performance studies under the IVDR?
We support companies in life sciences
Since 2011, QbD has been providing quality solutions for product development and manufacturing. The QbD Group’s team offers the skills and expertise for solving complex problems for companies active in the life sciences.

We make quality management easy
The first cloud-based and pre-validated Quality Management Software built for Life Sciences. Writing down your quality processes is one thing, but making sure everybody is aware of them and following-up on events, CAPAs and a plethora of related actions is what keeps QA people awake at night. With Scilife you can reclaim your peaceful nights again.
We support your full project in a flexible way
We work with a risk-based, project plan approach, focusing on what’s really important:
- increased efficiency
- reduced workload
- reduced total compliance costs
- guaranteed optimal quality, safety and compliance of products
Our project plans and services vary from short-term and long-term consultancy to a Quick Scan, taking care of complete projects or working together according to our unique Academy Model.


ATMPS ARE A REALITY
Who is the QbD Group?
By bringing our consultants and their expertise together with our life sciences clients in the best possible way, we help our clients to develop safe therapies and technologies for patients and consumers.
We do this worldwide, with over 550 people who are highly qualified in regulatory affairs, clinical, qualification & validation, quality assurance, quality control, software services & solutions and business & communication.
To this end, we have set up offices including Belgium (HQ), the Netherlands, France, Switzerland, Spain, Mexico, and Colombia.
Looking for a dream job in life sciences?
QbD Group’s dedicated life science consultants advise and support companies worldwide, from idea to patient. Up for a new challenge? Be sure to check our top jobs in life sciences and get in touch!
Latest company news
Latest blog posts
WE WORK, WE PLAY, WE DELIVER
Global reach with a local presence
QbD is an international organization. Not only because of its offices across the globe (Belgium, the Netherlands, Spain, France, Mexico, Colombia, and many more), but its global network enables QbD also to execute projects in many places.
Whitepapers
We are happy to share our knowledge with you via free to download content pieces.